15-02-2026
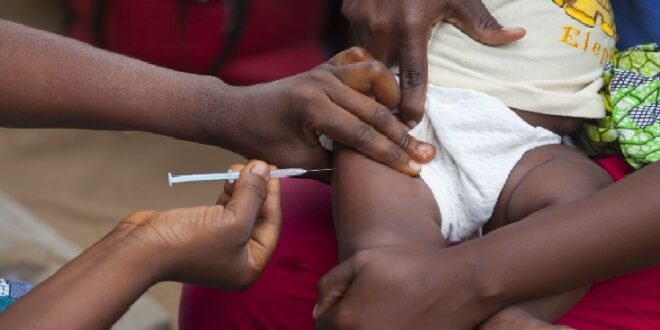
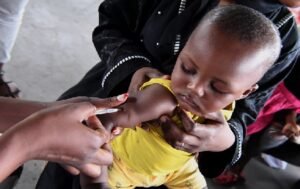
GENEVA/ BISSAU: The World Health Organization on Friday released a formal statement blasting a US-funded vaccine trial as “unethical,” because it would withhold an established, safe, and potentially lifesaving vaccine against hepatitis B from some newborns in Guinea-Bissau, Africa.
 “In its current form, and based on publicly available information, the trial is inconsistent with established ethical and scientific principles,” the WHO concluded, after providing a bullet-point list of reasons the trial was harmful and low quality.
“In its current form, and based on publicly available information, the trial is inconsistent with established ethical and scientific principles,” the WHO concluded, after providing a bullet-point list of reasons the trial was harmful and low quality.
The trial has drawn widespread condemnation from health experts since notice of the US funding was published in the Federal Register in December. The notice revealed that the Centers for Disease Control and Prevention under anti-vaccine Health Secretary Robert F. Kennedy Jr. had awarded $1.6 million to Danish researchers for their non-competitive, unsolicited proposal to conduct the trial.
The Danish researchers are led by Christine Stabell Benn and her husband Peter Aaby of the Bandim Health Project, which is based at the University of Southern Denmark in Copenhagen. Benn and her colleagues have long been controversial for their questionable practices in research into alleged vaccine safety concerns. Kennedy has cited their work in his decision to cut global vaccine funding.
The CDC’s funding of Benn’s hepatitis B vaccine trial also came soon after the agency’s advisors who were handpicked by Kennedy decided to abandon a long-standing universal recommendation for a hepatitis B vaccine birth dose. The move was widely decried by health experts.
In the Guinea-Bissau trial, Benn’s team intended to randomize 14,000 newborns to receive their first dose of hepatitis B vaccine at either birth or at six weeks, and then study differences in alleged safety outcomes. Currently, Guinea-Bissau gives the first vaccine dose at the six-week point, but has already decided to transition to recommend a birth dose in 2028. The reason for the delayed implementation is resource constraints.
“Exploiting scarcity is not ethical,” the WHO wrote in its statement today.
The United Nations health agency highlighted that the hepatitis B vaccine birth dose is “an effective, and essential public health intervention” that has “been used for over three decades, with more than 115 countries including it in their national schedules. “
“It prevents life‑threatening liver disease by stopping mother‑to‑child transmission at birth,” the WHO wrote, noting that more than 12 percent of adults in Guinea-Bissau have chronic hepatitis B.
In a section subtitled “Why withholding the vaccine is unethical,” the WHO lays out all the reasons the trial is dangerous.
“From what is publicly described, the (trial) protocol does not appear to ensure even a minimum level of harm reduction and benefit to the study participants (e.g., screening pregnant women and vaccinating newborns exposed to hepatitis B),” the WHO wrote.
As a proven lifesaving vaccine, withholding it from some study participants would expose newborns to serious and potentially irreversible harm, including chronic infection, cirrhosis, and liver cancer, the WHO argues. There is no scientific justification for withholding a proven intervention, and there is no credible evidence of the safety concerns that Benn and her colleagues claim to be looking for in their trial. The WHO also noted that the publicly available information about the trial indicates that it will be a single-blind, no-treatment-controlled design, which “raises a significant likelihood of substantial risk of bias, limiting interpretability of the study results and their policy relevance.” (Int’l News Desk)
 Pressmediaofindia
Pressmediaofindia