05-10-2025
Bureau Report
NEW DELHI/ HYDERABAD: Indian authorities said on Saturday they are investigating if contaminated cough syrup caused the deaths of nine children in a central state after a batch of the medication was found to contain dangerous levels of a toxic chemical.
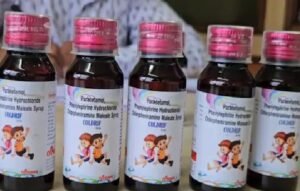
 The Health Ministry said samples of Coldrif Cough Syrup, manufactured by Sresan Pharma in the southern state of Tamil Nadu, were tested by state authorities and found to contain diethylene glycol (DEG) exceeding permissible limits.
The Health Ministry said samples of Coldrif Cough Syrup, manufactured by Sresan Pharma in the southern state of Tamil Nadu, were tested by state authorities and found to contain diethylene glycol (DEG) exceeding permissible limits.
“The samples are found to contain DEG beyond the permissible limit,” the ministry said in a statement.
DEG, a toxic solvent used in industrial products, has been linked to fatal poisoning in several countries.
The statement comes after media reports suggested that recent deaths of nine children in the central state of Madhya Pradesh could have been linked to cough syrup consumption.
The Madhya Pradesh Food and Drug Administration (MPFDA) also analyzed three of 13 samples collected, which were found free of contamination, the ministry statement said.
However, Tamil Nadu’s drug regulator later confirmed DEG contamination in samples taken directly from Sresan Pharma’s manufacturing site in Kanchipuram, it said.
Sresan Pharma did not immediately respond to a request for comment sent by email.
Authorities have launched inspections of 19 drug manufacturers across six states to identify quality control lapses and recommend improvements to prevent future incidents, the ministry said.
 India has faced scrutiny over the quality of its pharmaceutical exports after the World Health Organization linked cough syrups made by another company to the deaths of 70 children in Gambia in 2022, a finding that New Delhi later disputed.
India has faced scrutiny over the quality of its pharmaceutical exports after the World Health Organization linked cough syrups made by another company to the deaths of 70 children in Gambia in 2022, a finding that New Delhi later disputed.
Last year, India announced that the concerned authorities will take “appropriate action” after completing an investigation into a complaint that a drug regulator helped switch samples of cough syrup linked to the death of children in Gambia in return for a bribe, two officials said on Wednesday.
The World Health Organization (WHO) linked the syrups made by India’s Maiden Pharmaceuticals to the deaths of 70 children in 2022, though India’s government said subsequent tests at an Indian government laboratory showed the syrups were not toxic.
Maiden, whose factory is based in Haryana state, denies wrongdoing.
Media reported in June last year that a lawyer named Yashpal accused Haryana’s drug controller, Manmohan Taneja, of taking a bribe of 50 million rupees ($602,195) from Maiden to help switch the samples before they went for tests at the government laboratory. Taneja has denied the charges.
The investigator, Gagandeep Singh and Haryana FDA Commissioner Ashok Kumar Meena, told media their superiors would decide on the “appropriate action” on the matter “as per law”, declining to share their findings.
“We are very much clear that if anyone has done anything wrong, we will take strict action,” Meena said. “There’s zero tolerance against corruption. If any wrong thing is found, action will be taken, whether it is Taneja or anyone else.”
Taneja did not respond to a call and a message seeking comment outside business hours. He told media in October that the probe had been triggered by a “fake complaint from a fake person” and that “anyone can send any fake complaint against anyone”.
 Pressmediaofindia
Pressmediaofindia




