16-06-2024
NEW YORK/ HOUSTON: Blood-sugar monitoring devices could soon be on the arms of millions of Americans after regulators cleared two new devices for use without a prescription. Is it a way to improve our health? Or is the data just another distraction?
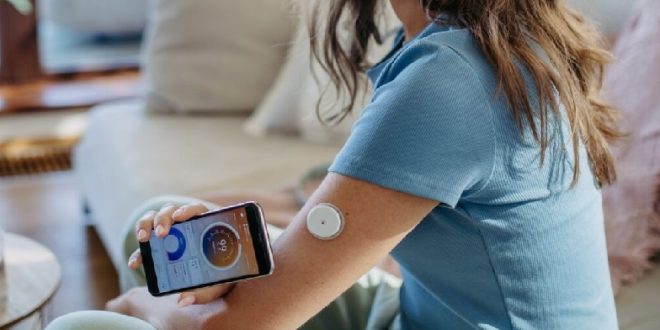
In the middle of the night last June, Cindy Bekkedam woke up to the sound of an unfamiliar alarm. It was loud, like an emergency alert, and it was coming from her phone. More specifically, it was coming from a newly installed app linked to a glucose sensor embedded in her arm.
 According to this app, her blood sugar had dropped to a concerning low while she slept, which had triggered the alarm.
According to this app, her blood sugar had dropped to a concerning low while she slept, which had triggered the alarm.
“So I got up in the middle of the night and ate a granola bar,” she said.
Continuous glucose monitors (CGMs), which monitor glucose levels in real time, have been used by millions of diabetics for years. As a dietitian in Ontario, Canada, Ms Bekkedam had hers installed to better understand the technology for her patients with diabetes but her two-week trial became somewhat of a cautionary tale.
“I was freaking out,” she said. “I actually questioned, oh my goodness, do I have diabetes?”
She didn’t and, after some extra research, she found that her glucose levels were entirely normal but constantly getting updates on her blood sugar highs and lows, without having a medical condition that required it, incited some unnecessary fear.
“That’s where I think people could go down a rabbit hole,” she said but these devices may be in the hands or on the arms of many more people very soon, thanks to two recent Food and Drug Administration (FDA) approvals for more widespread use. This week, Abbott Laboratories announced it had received federal clearance for two over-the-counter CGMs, including one for those without diabetes.
Use of CGMs is already rising, with the tell-tale arm patches easily spotted during morning commutes in major American cities but experts say that even if there is no proven harm, there is little evidence to warrant spending the hefty fees – as much as $300 (£240) a month if you’re not a diabetic.
 Abbott’s Lingo, which is a CGM for people without diabetes, is marketed to consumers “who want to better understand and improve their health and wellness”. It was one of two devices cleared by the FDA for sale and is already available in the UK. The FDA’s 510(k) regulatory process evaluates medical devices for safety and efficacy, but marketing claims are not part of the review.
Abbott’s Lingo, which is a CGM for people without diabetes, is marketed to consumers “who want to better understand and improve their health and wellness”. It was one of two devices cleared by the FDA for sale and is already available in the UK. The FDA’s 510(k) regulatory process evaluates medical devices for safety and efficacy, but marketing claims are not part of the review.
“Understanding your body’s glucose is key to managing your metabolism so you can live healthier and better,” an Abbott spokesperson told media.
Abbott said that flattening glucose curves could help improve energy, mood and sleep and pointed to studies showing the impact of glucose spikes on overall health, and the role of CGMs in monitoring them.
There is scepticism about such claims in the medical community but one thing experts agree on is that CGMs have significantly improved the care of some people living with diabetes.
Type 1 diabetes is when an individual’s pancreas stops producing insulin, so regular injections are needed. Type 2 diabetes is more common and occurs when the cells in the body become resistant to insulin and so more is needed to keep blood glucose levels within a normal range. It can usually be controlled through medication, diet, exercise and close monitoring, although some take insulin. Traditionally, diabetics monitored their blood sugar with finger-prick tests, but CGMs can alert people with diabetes when their blood sugars are running dangerously high and low, and if insulin needs to be injected. (Int’l News Desk)
 Pressmediaofindia
Pressmediaofindia